- INTRODUCTION
- DEFINITION OF AXES IN EARLY EMBRYOS
- POLARITY IN UNFERTILIZED EGGS
- POST-FERTILIZATION PROCESSES THAT ESTABLISH THE ANIMAL VS VEGETAL CELL FATES
- Cytoplasmic and Cortical reorganization during the first cell cycle
- À-catenin and Vegetal Cell Fate Specification
- POST-FERTILIZATION PROCESSES THAT SPECIFY THE ANTERO-POSTERIOR AXIS
- ZYGOTIC GENE EXPRESSION THAT ESTABLISH THE ANIMAL VSVEGETAL CELL FATES
- Zygotic Genes Downstream of À-catenin
- Zygotic Genes for Gastrulation and Endoderm Fate Specification
- CELL INTERACTIONS AND ZYGOTIC GENE EXPRESSION ALONG THE ANTERO-POSTERIOR AXIS
- Induction of Notochord and Mesenchyme
- FGF signaling
- Directed Signaling and Asymmetric Division
- Responsiveness to inductive signal
- Zygotic Expression of Key Transcription Factors ? Muscle and Notochord
- Zygotic Expression of Key Transcription Factors ? Mesenchyme, TVC, and TLC
- Animal Hemisphere
- SUMMARY AND FUTURE DIRECTIONS
- REFERENCES
CONTENTS
INTRODUCTION
The three-dimensional structure of organisms is characterized by the three perpendicular body axis, namely, the anterior?posterior, dorsal?ventral, and left?light axes. These axes generally emerge in the early embryonic stage, and are sometimes already apparent in eggs before fertilization. One of the major issues in developmental biology is to understand how axial polarity is achieved in a developing embryo. The ascidian embryo is a classical model that has been used to study axis and cell fate specification during embryogenesis for over a century (Chabry, 1887; Conklin, 1905; reviewed by Satoh, 1994; Nishida, 1997; Jeffery, 2001). Recent advances in ascidian research have placed this simple organism in a unique position to contribute to a detailed understanding of the cellular and molecular processes of embryogenesis (reviewed by Corbo et al., 2001; Nishida, 2002a, b; Satoh, 2003a, b). Most of recent concepts and analysis of ascidian development derive from observations and experimental works using four main evolutionarily distant species (Ciona intestinalis and savignyi, Halocynthia roretzi, and Phallusia mammillata), which have contributed complementary and/or converging or diverging information.
The restriction of cell fate proceeds quickly in ascidian embryos. Just before gastrulation is initiated at the 110-cell stage, the developmental fates of most blastomeres are restricted such that a blastomere gives rise to a single cell type in the tadpole larva (Fig. 1) (Nishida, 1987). Even when blastomeres are isolated from 110-cell embryos, they continue to develop into differentiated cells according to the fate map (Reverberi and Minganti, 1946; Nishida, 1992a). This intriguing cell-autonomous property of ascidian embryos is referred to in many textbooks as a typical example of mosaic development. Therefore, it will be sufficient to take into account the events that happen before the 110-cell stage in order to fully understand axis specification in this animal. This article summarizes the current knowledge on maternal and zygotic processes related to embryonic axis specification in ascidians. In this article, we first describe post-fertilization processes involving maternal factors before zygotic gene expression is initiated. Then processes that require zygotic gene expression and cellular interactions after embryos become multicellular will be summarized.
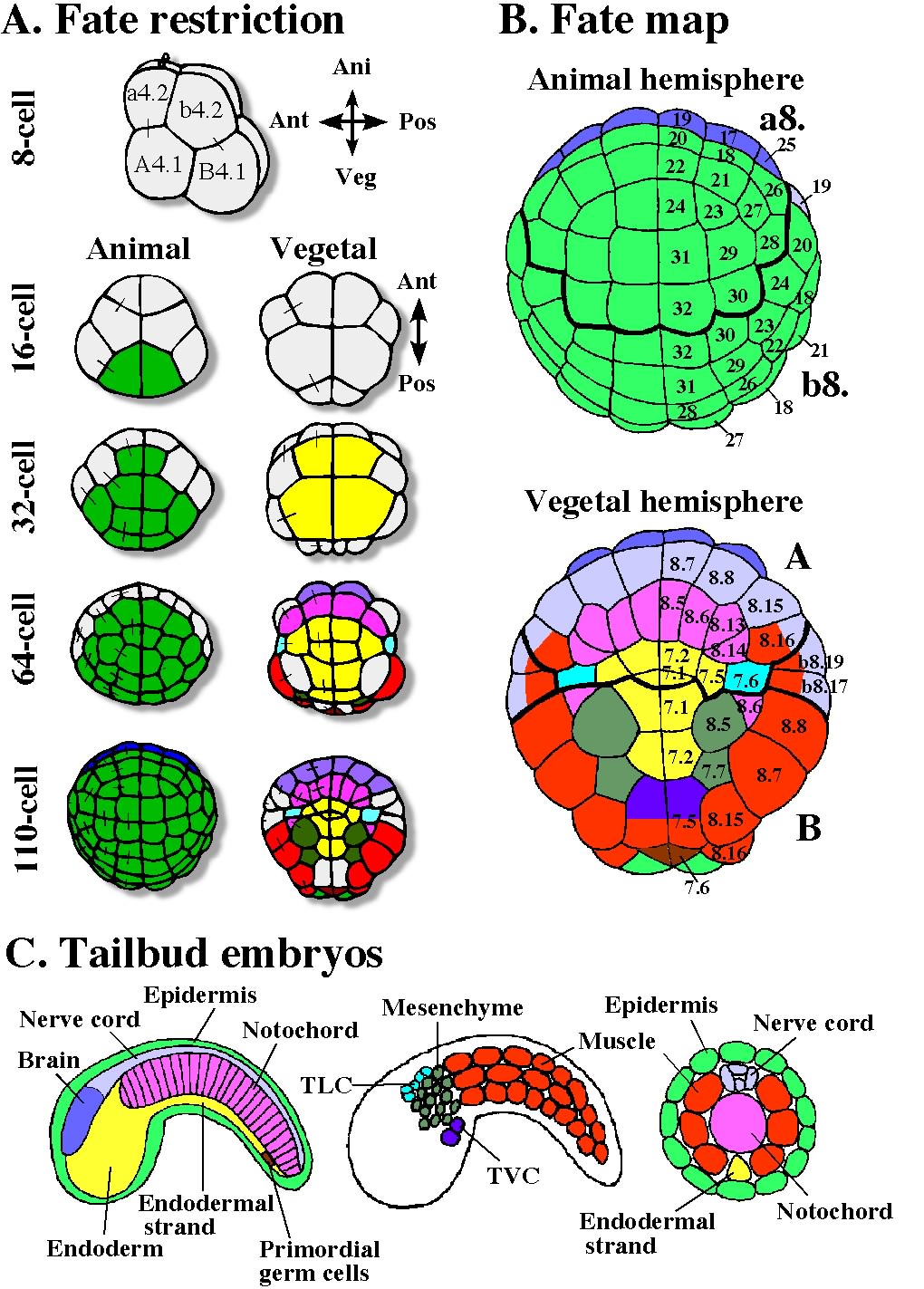
Fig. 1. Developmental fates of blastomeres of the ascidian embryo. A: Fate restriction during cleavage stages. Blastomeres are colored when the fate of the blastomere is restricted to give rise to a single kind of tissue cell. The colors correspond to the colors of larval tissues indicated in C. Fate restriction in ascidian embryos proceeds quickly in the early embryo. Sister blastomeres of the previous cleavage are connected by bars. B: Fate map of the 110-cell stage. Animal and vegetal hemispheres. Names of blastomeres are indicated as ea8.19f etc. C. Organization of tailbud embryos. Mid-sagittal planes, sagittal planes, and transverse sections of the tail.
DEFINITION OF AXES IN EARLY EMBRYOS
The definition of embryonic axis in early embryos is somewhat artificial and is a question of semantics. It is difficult per se to superpose future embryonic axes on eggs and embryos before and during gastrulation, because dynamic movements and rearrangements of cells occur in gastrulae. Differences in axis definition between amphibians and ascidians (which show similar morphogenetic movement) provide an example of the difficulties (Fig. 2). In ascidians, the animal?vegetal (A?V) axis has conventionally been considered to be the future ventral?dorsal (V?D) axis, and the axis perpendicular to the A?V axis is the anterior?posterior (A?P) axis (Conklin, 1905). By contrast, in Xenopus, the definition of embryonic axis is the opposite. The A?V axis is assigned as the future A?P axis, and the D?V axis is perpendicular to it (Nieuwkoop, 1977). The difference comes from the difference in the position of the developing embryo used as a reference point (Arendt and Nubler-Jung, 1997). In this sense, the definition of axes in early embryos is somewhat artificial. In studies of ascidians, the animal pole, indicated by the site of polar body extrusion, is always held upward. As axial structures such as notochord and nerve cord undergo convergent and extension movement, and thus the dorsal blastopore lip of ascidian gastrulae heads vegetally, the original vegetal pole is covered by these dorsal tissues. This is why the vegetal pole corresponds to the future dorsal side in ascidians. Looked at this way, embryos lie in a somewhat unusual way on their back. This may not matter to ascidian researchers because ascidian embryos develop in any orientation in seawater like sea urchin embryos. In contrast, in a usual textbook drawing of amphibian gastrulation (e.g. Gilbert, 2003), the dorsal blastopore lip is held in a constant position. In this presentation, one holds the region of dynamic cell movement and actually holds no cell constantly. With this as a fixed point it is necessary to turn other regions around such as the regions with less dynamic cell movement, for example, animal pole cells. This presentation makes sense in light of the fact that amphibian embryos settle and are oriented by gravity. When one draws gastrulating embryos of either animal in another way, one can see how morphogenetic movements are conserved between ascidians and amphibians.
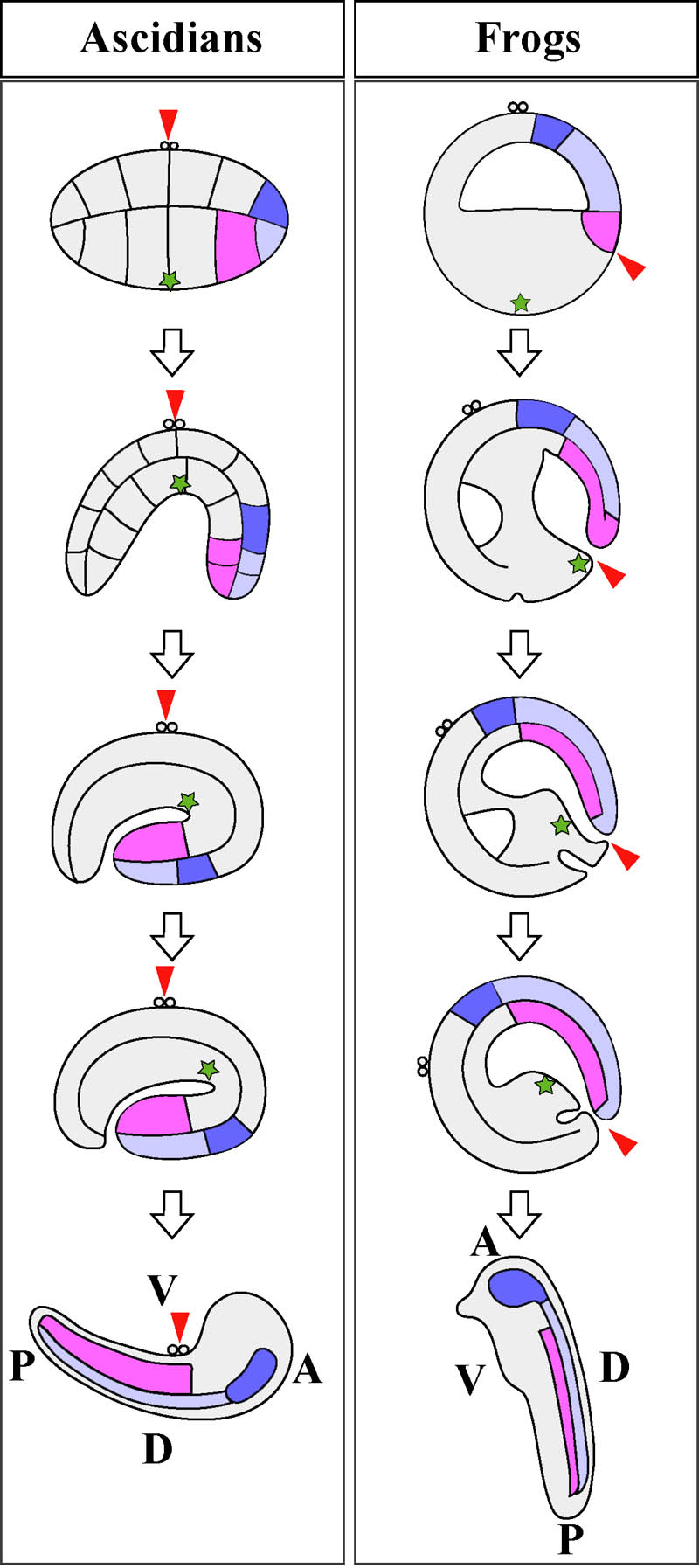
Fig. 2. Schematic representation of morphogenetic movements during gastrulation of ascidians (left) and frogs (right). Presumptive notochord region is colored pink. Anterior and posterior neural tube is colored light and dark blue. Polar bodies indicate the animal pole. Green asterisks point the original vegetal pole. Red arrowhead represents the point held in a constant position. Embryonic axes at tailbud stage are indicated at the bottom. See text in detail.
In ascidians, the A?P axis of the early embryo (corresponding to the D?V axis in amphibians) is defined as perpendicular to the A?V axis, as the animal pole represents the reference point. But it does not correspond precisely to the A?P axis of larvae, because cells do not remain stationary during morphogenetic movements. Complicated positional rearrangement does not take place in the animal hemisphere and animal cells just cover the embryo to form ectoderm by epiboly, so the A?P axis of eggs and early embryos in the animal hemisphere corresponds precisely to the A?P axis of larvae. Anterior-animal blastomeres give rise to head/trunk epidermis of larvae, and posterior-animal blastomeres develop into tail epidermis. The polar bodies attach to the ventral midline at the boundary of trunk and tail of the tadpole (Fig. 2). What happens in the vegetal hemisphere is not so simple (Fig. 1). Anterior-vegetal blastomeres give rise mainly to endoderm, notochord, and nerve cord. Notochord and nerve cord cells are located in the larval tail. Posterior-vegetal blastomeres develop mainly into endoderm, mesenchyme, and muscle. Muscle cells are found in the tail, while endoderm and mesenchyme cells are found in the trunk region. In the present paper, ganteriorh and gposteriorh are used in the way these adjectives are traditionally applied to ascidian eggs and early embryos. Thus, in the present context, ganterior fateh is that of blastomeres of the anterior half of cleavage-stage embryos and does not mean the fate that gives rise to the anterior trunk structures of larvae, nor does gposterior fateh mean the fate that gives rise to the posterior tail structures.
¢Back to CONTENTSPOLARITY IN UNFERTILIZED EGGS
Generally the A?V axis is already present in unfertilized eggs. The axis in pre-gastrula embryos is correlated with germ layer segregation. In ascidians, ectoderm, mesoderm, and endoderm territory is present along the A?V axis, as in amphibian (Fig. 1). Processes that involve maternally stored factors and occur before the activation of the zygotic genome are discussed in this and following two chapters. The mature oocytes of most animals show radial symmetry with a single A?V axis. The animal pole is where polar bodies form during meiosis. Unfertilized eggs of ascidians are in meiotic metaphase I with the meiotic spindle at the animal pole. In addition to the spindle, ascidian eggs already show various kinds of polarization along the A?V axis in the subcortical and cortical domains (Fig. 3). No remarkable polarization in the central egg cytoplasm has been reported. In the subcortical region, mitochondria are enriched in the vegetal half forming a mitochondria-rich region, called the myoplasm, from which microtubules are excluded (Zalokar and Sardet, 1984). The localization of mitochondria in myoplasm would have an adaptive significance in that they are preferentially segregated into larval muscle cells, which need vigorous energy production. In the egg cortex, microfilaments form a basket with its opening at the animal pole region (Sardet et al., 1992). This gives a mechanical motive force for the first phase of cytoplasmic and cortical reorganization, called ooplasmic segregation, as mentioned two paragraphs below. A network of tubules and sheets of cortical endoplasmic reticulum (cER) is present just beneath the plasma membrane, showing a gradient with the highest density at the vegetal pole. The cER is tethered to the plasma membrane (Sardet et al., 1992).
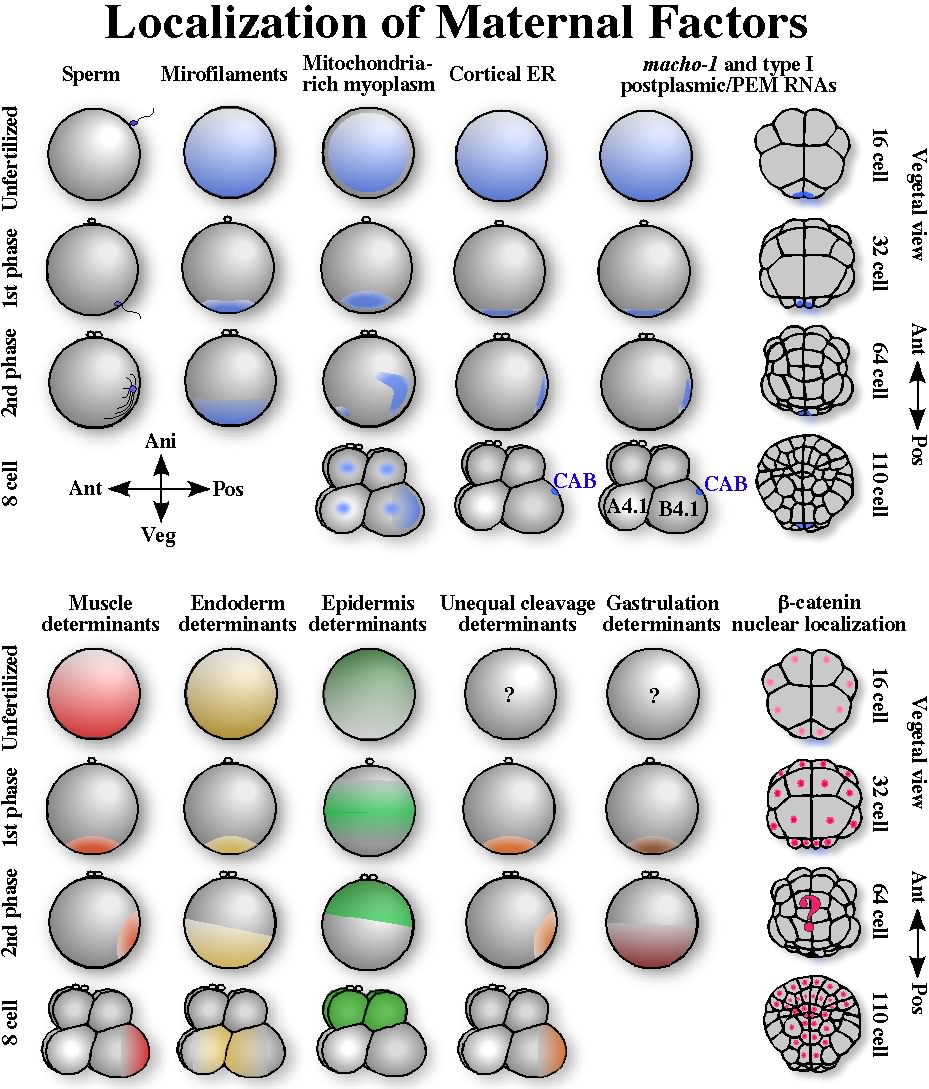
Fig. 3. Localization of maternal factors from fertilization to the 110-cell stage. Cytoplasmic and cortical reorganization (ooplasmic segregation) proceeds in two major phases during the first cell cycle. The centrosome-attracting body (CAB) is present in the posterior region of the B4.1 blastomeres of the 8-cell embryos, and is inherited by the posterior-most blastomeres during cleavages. Cortical ER and postplasmic/PEM RNAs are concentrated into the CAB. The bottom part shows localization of various maternal determinants as inferred from ooplasmic removal and transplantation experiments. Nuclear localization of ?-catenin during cleavages is shown at the bottom-right.
In addition to these membranous and cytoskeletal components, several maternal mRNAs, called type I postplasmic RNAs in Halocynthia (Sasakura et al., 2000) or PEM (posterior end mark) RNAs in Ciona (Yoshida et al., 1996), show a polarized distribution with the highest concentration at the vegetal pole cortex (Fig. 3) (reviewed in Nishida, 2002a; Sardet et al., 2005). These type I postplasmic/PEM RNAs encode a great variety of protein types. They show a polarized distribution in the unfertilized egg cortex, but their role in the establishment of the A?V axis is unclear. Some of them are clearly involved in the formation of the A?P axis, as their final destination after ooplasmic movements is the posterior region, as discussed in the next section. The results of transplantation of the peripheral cytoplasm have also revealed the polarized distribution of a tissue-forming substance in unfertilized eggs. Muscle and endoderm forming activities are present in a gradient fashion with the highest activity at the vegetal pole, while epidermis forming activity is present in an opposite gradient (Nishida, 1992b; Nishida, 1997, and references therein) (Fig. 3). Thus, unfertilized eggs already show apparent polarity, however, when and how the polarity is established during oogenesis and oocyte maturation is unknown. In Halocynthia oocytes, the germinal vesicle is in an eccentric position (Numakunai, 2001), but this does not seem to be the case in Ciona and Phallusia oocytes.
¢Back to CONTENTSPOST-FERTILIZATION PROCESSES THAT ESTABLISH THE ANIMAL VS VEGETAL CELL FATES
Cytoplasmic and Cortical reorganization during the first cell cycleEggs of many kinds of animal show cortical and cytoplasmic movements just after fertilization. These processes have been extensively analyzed in C. elegans, Xenopus, and ascidians. The mechanisms involved and factors translocated are diverged among these animals, however, egg cortices commonly play a significant role. The cortex provides a scaffold to anchor and translocate localized maternal factors, and supplies spatial information for later embryogenesis (reviewed by Sardet et al., 1994, 2002). Ascidian eggs undergo dramatic cytoplasmic and cortical reorganizations between fertilization and the beginning of the first cleavage; this process has been called ooplasmic segregation (Fig. 3) (Conklin, 1905; Sardet at al., 1989; Roegiers et al., 1999). Reorganizations occurs in two major phases. Shortly after fertilization (0?10 min after insemination in Halocynthia, 0-5 min in Ciona and Phallusia), the first phase of ooplasmic segregation is accompanied by a rapid contraction of the egg cortex and plasma membrane, resulting in a segregation of microfilaments, mitochondria, cER, postplasmic/PEM RNAs, and muscle- and endoderm-forming activity towards the vegetal pole region (Roegiers, et, al., 1999; Nishida, 1997; Sardet et al., 2003). An microfilament basket with its opening at the animal pole contracts toward the vegetal pole, bringing these components toward the vegetal pole region. Eggs still show radial symmetry along the A?V axis at this stage, but the original polarity is much intensified, as all components mentioned above are highly concentrated at the vegetal pole region (Fig. 3). In Halocynthia, the contraction pole is at the opposite position of the polar bodies in most cases. However in Phallusia and Ciona, the contraction pole can be off the opposite position by as much as 45-60 degree depending on the site of the sperm entry (Roegiers et al., 1995), making it difficult to define the A-V axis unambiguously.
The second phase of reorganization (85?110 min in Halocynthia, 25-45 min in Ciona and Phallusia) brings most of these components toward the future posterior pole (Fig. 3) (Sawada, 1988; Sardet et al., 1989; Nishida, 1997; Roegiers et al., 1999), and is relevant to specification of the A?P axis. Exceptionally, factors involved in endoderm formation and gastrulation do not move toward the future posterior pole, and just expand their distribution to the entire vegetal hemisphere during the second phase (Fig. 3) (Bates and Jeffery, 1987; Nishida, 1996). The significance of this second phase in the A-P axis specification is discussed in the next chapter.
À-catenin and Vegetal Cell Fate SpecificationRecently, molecular aspects of post-fertilization processes that is involved in establishment of the A?V axis in sea urchin embryos have been intensively investigated. Dishevelled (Dsh), glycogen synthetase kinase 3 (GSK-3), and ?-catenin are involved. These molecules are signaling molecules in the canonical Wnt pathway. The Wnt signaling pathway is generally utilized in cellular communication. However, early embryos utilize only a downstream part of the intracellular signal transduction cascade in maternal mechanisms that specify embryonic axes. In amphibian and fish embryos, this maternal system specifies the D?V axis, which is perpendicular to the A?V axis (Miller and Moon, 1996; Heasman, 1997; Schneider et al., 1996; Miller et al., 1999). However, the same pathway is utilized to establish the A?V axis in echinoderms (Wikramanayake et al., 1998; Emily-Fenouil et al., 1998; Logan et al., 1999; Weitzel et al., 2003). The role of this pathway in germ layer segregation would be conserved among ancient metazoans, as Cnidaria embryos also shows nuclear À-catenin in cells that invaginate (Wikramanayake et al., 2003). In the canonical Wnt signaling pathway, Wnt proteins bind to the transmembrane receptor Frizzled and then activate Dsh, which in turn inhibits GSK-3 activity. GSK-3 destabilizes À-catenin protein. Hence, inhibition of GSK-3 activity results in À-catenin stabilization and its translocation into nuclei. À-catenin forms a complex with the transcription factor Tcf to activate specific gene expression (Cadigan and Nusse, 1997). In the sea urchin, nuclear accumulation of À-catenin occurs in the vegetal region of cleaving embryos, and this is a crucial event in endomesoderm (vegetal cell fates) specification. Dsh is required for the stabilization of À-catenin in vegetal cells. GFP-tagged Dsh protein is targeted to the vegetal cortex of the unfertilized egg (Weitzel et al., 2003). Therefore, Dsh seems a key localized protein, although the authors argue the possibility that Dsh activator is also localized in the vegetal cortical region, because overexpressed Dsh mRNA cannot promote vegetal fate in animal blastomeres.
In ascidians, Imai et al. (2000) reported a role for À-catenin in vegetal fate specification in Ciona intestinalis and C. savignyi embryos. In this regard, ascidian embryos show a similarity to echinoderm embryos. Preferential À-catenin nuclear localization occurred in the vegetal hemisphere in cleavage-stage embryos (Fig. 3). When mRNA encoding the stabilized form of À-catenin (lacking the phosphorylation sites for GSK-3) was injected into eggs, nuclear À-catenin was also observed in the animal hemisphere. In these embryos, most embryonic cells produced alkaline phosphatase, an endoderm differentiation marker, and the expression of an epidermis marker, Cs-Epi1, was downregulated. The only exception concerned primary muscle precursor cells, whose fate was not altered by the overproduction of À-catenin. To inhibit À-catenin function in nuclei, À-catenin was sequestered into a cell adhesion complex by overproduction of cadherin. In these embryos, nuclear staining with À-catenin antibody was abolished in the entire embryo. Alkaline phosphatase expression was lost, and Cs-Epi1 expression expanded into the vegetal hemisphere. The embryos did not gastrulate. The development of muscle cells was not perturbed in these cadherin-overproducing embryos. Neither À-catenin mRNA nor protein was localized to the vegetal cytoplasmic region of eggs and initial cleavage stage embryos. This suggest that the localized endoderm determinants may be molecules that stabilize À-catenin in the vegetal hemisphere. A promising candidate is Dsh, as has been recently shown in sea urchins. Identification of maternal endoderm determinants and gastrulation determinants in ascidians remains elusive for the moment.
These observations indicate that A?V axis establishment is mediated by À-catenin signaling. Endoderm (vegetal) fate requires À-catenin function, while epidermis (animal) fate is promoted by suppressing À-catenin function. It is not known whether mesoderm cells in the marginal zone of the vegetal hemisphere, such as notochord and mesenchyme precursors, require intermediate stabilization of À-catenin within the cells or not. In sea urchin, there is a gradient in À-catenin stability along the A?V axis during early cleavage, and this gradient could be involved in specifying an intermediate zone along the A?V axis (Weitzel et al., 2003). In ascidians, the presence of such a gradient has not yet been investigated.
In Xenopus, maternal VegT mRNA is localized to the vegetal pole of the egg. VegT encodes a T-box transcription factor and is required for specification of the endomesoderm germ layer in the vegetal hemisphere (Zhang et al., 1998). However, in Ciona, there are seven T-box transcription factor genes (one of these is present as triplicate copies) in the genome, and none of their maternal mRNAs are localized to the vegetal pole (Takatori et al., 2004). This is consistent with the observation that mechanisms involved in specification of vegetal fates may differ between echinoderms/ascidians and amphibians.
¢Back to CONTENTSPOST-FERTILIZATION PROCESSES THAT SPECIFY THE ANTERO-POSTERIOR AXIS
Cortical MovementsUnfertilized eggs and eggs after the first phase of ooplasmic segregation are radially symmetrical along the A-V axis. This symmetry is broken during the second phase of ooplasmic segregation, and eggs become bilaterally symmetrical before the first cleavage, as cortical and subcortical components, such as postplasmic/PEM RNAs that are located in the vegetal pole region after the first phase, move toward the future posterior pole (Fig. 3). This movement is directed by the centrosome introduced by the sperm and depends on the sperm entry point (Sardet at al., 1989; Roegiers et al., 1995). In Phallusia, the sperm tends to bind to the egg surface at the animal hemisphere (Speksnijder et al., 1989) and is conveyed partway toward the vegetal pole when the plasma membrane and cortex contracts during the first phase, which occurs shortly after fertilization. In contrast to this first phase, which is driven by microfilaments, the motive power for the second phase is principally generated by microtubules of the sperm aster (Sawada and Schatten, 1988). Vegetally located cortical components are then brought to the future posterior pole together with the sperm aster, which goes back just beneath the plasma membrane toward the equatorial region during the second phase. The male pronucleus and sperm aster never cross or go to the opposite side of the vegetal pole; therefore, the future posterior side coincides with the side of sperm entry point.
Role of the Posterior-Vegetal Cytoplasm/Cortex and Postplasmic/PEM RNAsThe posterior-vegetal cytoplasm/cortex (PVC) after the second phase of segregation and before the first cleavage plays crucial roles in specification of the A?P axis. Removal of the PVC (less than 10% of total egg volume) results in radialization of the cleavage pattern, producing a mirror image duplication of the anterior cleavage pattern in the original posterior half. It has an effect not only on the cleavage pattern, but also on the developmental fates of blastomeres, as they also show mirror image duplication of the anterior half. Transplantation of the PVC to the anterior region brings about mirror image posteriorization of the original anterior half (Nishida, 1994). It is worth noting that removal and transplantation of regional egg cytoplasm other than the PVC has no effect on embryogenesis. These micromanipulative experiments demonstrate that some important factors are localized to the PVC, and that no developmentally important factor is localized to any small area other than the PVC.During the second phase, cortical ER (cER) and subcortical mitochondria relocate posteriorly (Fig. 3). In contrast, the plasma membrane and cortical actin cap do not move. In the last decade, several maternal mRNAs that are localized to the PVC have been identified. The first example is the pem (posterior end mark) mRNA in Ciona savignyi, which was isolated using differential screening techniques with egg fragments prepared by centrifugation (Yoshida et al, 1996). Since then, nine mRNAs localized to the PVC have been identified in Halocynthia roretzi (macho-1, Hr-PEM, Hr-PEM-3, Hr-Wnr-5, Hr-POPK-1, Hr-GULT, Hr-PEN-1, Hr-PEN-2, Hr-ZF-1) (reviewed in Nishida, 2002a; Sardet et al., 2005) by the collaborative MAGEST project (cDNA project of maternal mRNAs: http://www.genome.jp/magest/) (Sasakura et al., 1998a, b, 2000; Makabe et al., 2001; Nakamura et al, 2003) and by subtraction hybridization screening between animal and vegetal blastomeres (Nishida and Sawada, 2001). These RNAs have been called type I postplasmic RNA in Halocynthia (Sasakura et al., 2000). They are present in cortex of the PVC. Some of them (macho-1, Hr-PEM) have been shown to be anchored to and segregate with the cER (Sardet et al., 2004). The cER network present in unfertilized egg provides a scaffold to anchor these maternal RNAs, and the second phase of ooplasmic segregation brings this cER/mRNA domain into an asymmetric position along the A?P axis. Localization sequence elements of some postplasmic RNAs were identified in their 3?UTRs (Sasakura and Makabe, 2002). Every type I postplasmic mRNA shows identical regional localization during ooplasmic segregation and cleavage stages (Fig. 3). They encode variety of protein such as transcription factors, secreted signaling protein, kinase, RNA-binding protein, and proteins with no obvious similarity to known proteins.
What are the functions of type I postplasmic/PEM RNAs? Removal and transplantation of the PVC of fertilized eggs, where type I postplasmic RNAs are localized, and other observations of events unique to the posterior region of developing embryos indicate the following possible functions. The suppression of functions of some of the postplasmic RNAs by antisense oligonucleotides supports their roles in posterior specification.
- 1. Muscle specification. A recent molecular study identified macho-1 mRNA as the localized maternal determinant of muscle formation in ascidians (Nishida and Sawada, 2001; Satou et al., 2002a). macho-1 mRNA is a type I postplasmic RNA, and encodes a Zn-finger protein that is transcriptional activator (Sawada et al., 2005). Without macho-1 function, muscle blastomeres assume nerve cord fate (Fig. 1) (Kobayashi et al., 2003).
- 2. Responsiveness to inductive signals. macho-1 is also involved in generating difference in responsiveness to inductive signals between notochord and mesenchyme precursor blastomeres. macho-1 confers responsiveness to induction of mesenchyme fate by extracellular fibroblast growth factor (FGF) signal on presumptive mesenchyme blastomeres (Kobayashi et al., 2003), as discussed later. Thus, macho-1 localization supports central roles in the specification of cell fates of the posterior blastomeres.
- 3. Unequal cleavage. The centrosome-attracting body (CAB) was originally discovered as a subcellular structure involved in generation of unequal cleavages (Hibino et al., 1998; reviewed in Nishida et al., 1999). During unequal cleavages in the posterior-most blastomeres, microtubule arrays extending from the posterior centrosome focus on the CAB, which is present just beneath plasma membrane at the posterior pole of the cleaving embryo. Then, as the microtubule arrays shorten, the interphase nucleus with the centrosome shifts posteriorly and approaches the CAB. Consequently, an asymmetrically located mitotic apparatus is formed. Unequal division then takes place, producing a smaller daughter cell that inherits the CAB in the posteriormost position in the vegetal half of the 16-cell embryo. This process takes place three times from the 8- to 64-cell stages, resulting in three successive unequal cleavages only at the posterior pole. These unequal cleavages make the cleavage pattern of the posterior-vegetal region unique, and different from the radial anterior cleavage pattern. The CAB in not present in eggs. The PVC of fertilized eggs is required and sufficient for the formation of the CAB and consequent unequal cleavages (Nishikata et al., 1999). Results of knockdown of Hr-PEM mRNA function in Halocynthia suggest that Hr-PEM is involved in the positioning of cleavage planes and unequal cleavages (Negishi, T., Sawada, K. and HN, unpublished).
- 4. Concentration of postplasmic/PEM RNAs into the CAB. Interestingly, all postplasmic RNAs are highly concentrated into a very restricted posterior region of the posterior-vegetal (B4.1) blastomere pair by the 8-cell stage. This region is indeed the CAB. The CAB is rich in ER (Iseto and Nishida, 1999), and cER bearing postplasmic RNAs in eggs is likely to be concentrated into the CAB during early cleavages (Sardet et al., 2003). During three rounds of successive unequal cleavages, the mRNAs are segregated into the smaller daughter cells located at the posterior pole together with the CAB (Fig. 3). Thus, the CAB serves as the core structure of a multifunctional complex that operates cleavage planes and anchors postplasmic RNAs. Having both functions together, the CAB assures that postplasmic RNAs are infallibly partitioned into one of the daughter cells after cell divisions.
- 5. Formation of primordial germ cells. An electron microscopic study of 16-cell embryos revealed that the CAB is also characterized by an electron-dense matrix that resembles the germ plasm in other animals (Iseto and Nishida, 1999). A pair of the posterior-most and smallest blastomeres (B7.6 cell pair, brown in Fig.1) of the 64-cell embryos that inherit the CAB cease to divide during early embryogenesis and give rise to two cells in the endodermal strand of the tadpole (Nishida, 1987). In the posterior-most blastomeres, zygotic gene expression is repressed (Tomioka et al., 2002), as observed in Drosophila and C. elegans germline cells (Seydoux and Dunn, 1997; Van Doren et al., 1998). The maternal vasa (Ci-DEAD1) mRNA is concentrated in the B7.6 cells in Ciona, and vasa protein also appears in the descendant cells at tailbud stage (Fujimura and Takamura, 2000; Takamura et al., 2002). After metamorphosis, Vasa-positive cells are found in the developing gonad. Vasa is a well known marker of germ cells in many animals (Ikenishi, 1998). These observations support the view that B7.6 cells are primordial germ cells in ascidians, and that the CAB, enriched in postplasmic/PEM RNAs, contains germ plasm.
- 6. Specification of trunk ventral cell precursors. Precursor blastomeres of trunk ventral cells (B7.5 cells, sister cells of B7.6 of the 64-cell embryo, purple in Fig. 1) form in the posterior region of early embryos (Nishida, 1987). Trunk ventral cells of tadpole larvae are progenitors of body wall muscle and heart of metamorphosed juveniles (Hirano and Nishida, 1997; Davidson and Levine, 2003). Recently, it was revealed that a Mesp ortholog in ascidians is specifically and transiently expressed in B7.5 cells (Fig. 4), and is essential for the specification of heart precursor cells (Satou et al., 2004). Maternal macho-1 at least is required for Mesp expression. Other postplasmic/PEM RNAs might be involved in the specification of the trunk ventral cells and zygotic expression of Mesp gene.
- 7. Control of cell cycle length. Cell divisions are retarded in the posterior-vegetal blastomeres compared to blastomeres in the other regions (Conklin, 1905). Postplasmic/PEM RNAs might be also involved in this retardation of cell cycle.
These events that are unique to the posterior region and have not been well analyzed yet, could be attributed to the postplasmic RNAs that also have not been analyzed. It is not known whether the A?P polarity of eggs is accounted for only by the asymmetric distribution of the postplasmic/PEM RNAs, although following discussion supports this possibility. Several postplasmic RNAs have been found as described above, but no maternal mRNA localized to other regions of eggs has been reported so far. This coincides with the results of removal and transplantation of egg cytoplasm other than the PVC.
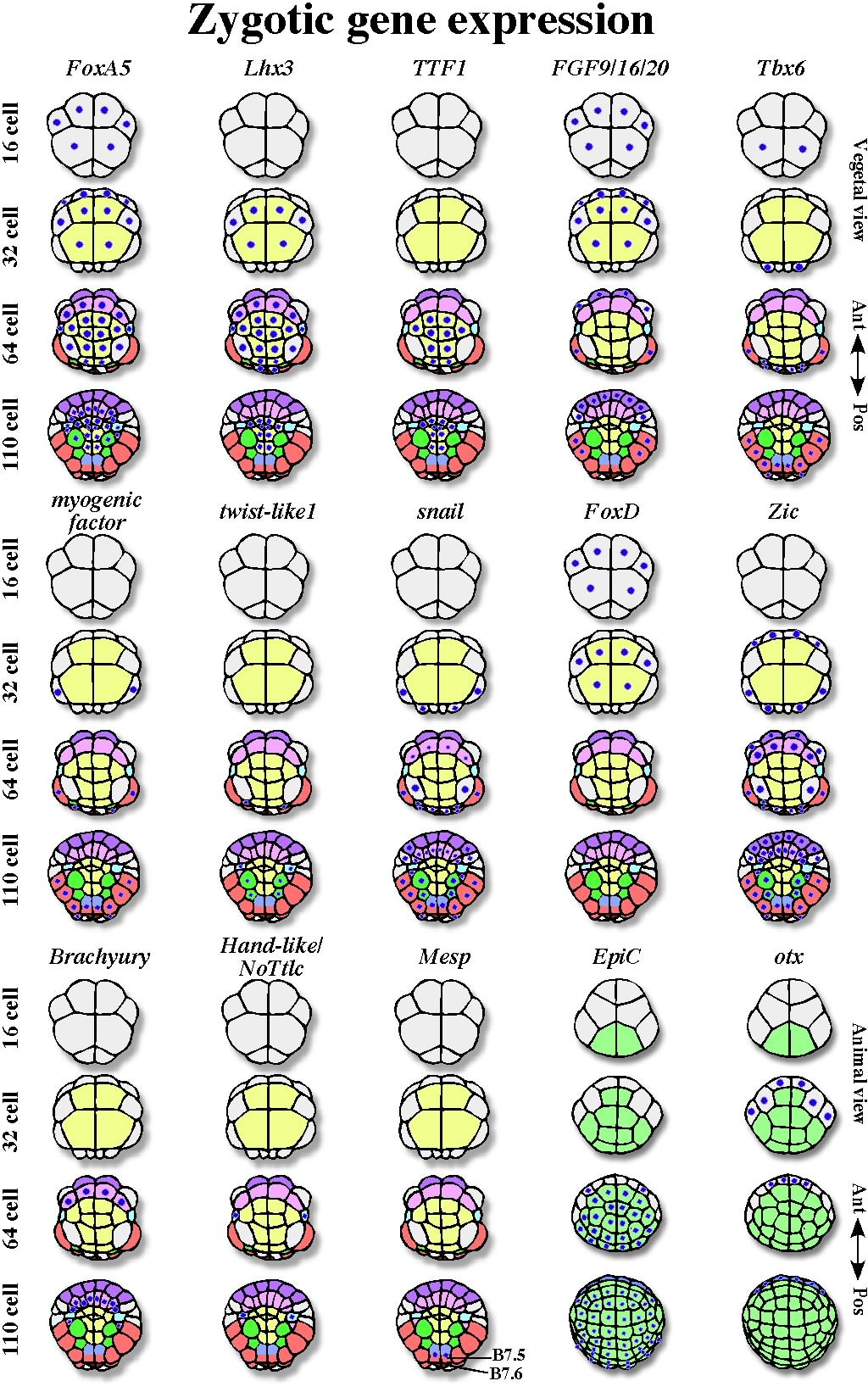
Fig. 4. Zygotic gene expression of various developmentally important genes during cleavage stages. Expression of each gene is indicated by blue dots on blastomeres. The light colors of blastomeres correspond to the colors used in the fate map shown in Figure 1. There are some differences in the expressions of Tbx6, snail, and Zic genes between Ciona and Halocynthia, although the differences are trivial and not relevant to the discussion in the review.
ZYGOTIC GENE EXPRESSION THAT ESTABLISH THE ANIMAL VSVEGETAL CELL FATES
Zygotic Genes Downstream of À-cateninWhen embryos become multicellular, cell interactions and zygotic gene expression start. So far, the earliest zygotic expression in ascidian has been observed from the 8-cell stage. Then expression of various genes starts at the 32-cell stage, corresponding to the mid-blastula stage in ascidians (e.g. Miya and Nishida, 2002). Several key transcription factors that show tissue-specific zygotic expression and are essential for the formation of each tissue have been identified in ascidians in this decade, as described below (Table 1).
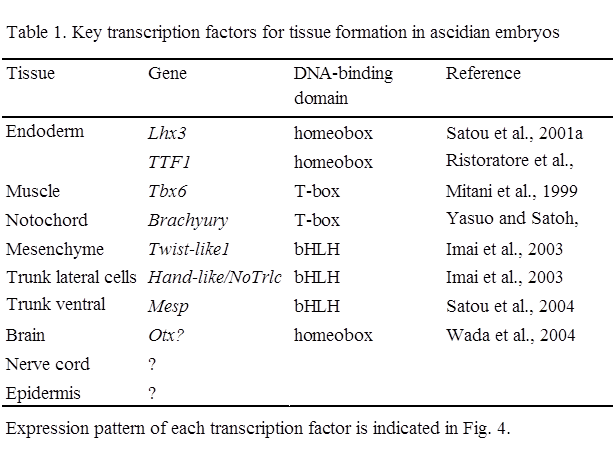
Nuclear À-catenin, together with transcription factor Tcf, promotes zygotic expression of various genes in the vegetal hemisphere. Genes downstream of À-catenin have been surveyed in Ciona by subtraction between embryos overproducing À-catenin and those overproducing cadherin (Satou et al., 2001a; Imai, 2003), and by comparison of expression levels by real-time RT-PCR for every transcription factor and signaling molecule in the Ciona genome in early gastrulae injected with a À-catenin morpholino antisense oligonucleotide (MO) (Imai et al., 2004). The downstream genes thus identified can be classified into three groups. One group represents genes that are involved in endoderm formation (FoxA5, Lhx3, TTF1). The second group includes genes that are relevant to embryonic induction of mesodermal tissues (Fgf9/16/20, chordin, FoxD, Zic, Brachyury, twist-like1, Mesp). The genes in the second group are discussed in the next chapter. Third group contains genes whose functions have not been fully analyzed in ascidians (cadherinII, protochadherin, Eph, lefty, dkk, DMRT1, hairy, ELK, Fli, jun, msxb, etc.). Essentially, the expression of these genes starts during cleavage stages in the vegetal hemisphere.
Zygotic Genes for Gastrulation and Endoderm Fate SpecificationThe FoxA5 (former forkhead/HNF3?) gene is expressed in vegetal blastomeres as early as the 16-cell stage (Mocu-FH1, Olsen and Jeffery, 1997; Hr-HNF3, Shimauchi et al., 1997; Ci-fkh, Di Gregorio et al., 2001). Its expression is rather broad in the vegetal hemisphere (Fig. 4). When antisense oligo DNA interfered with the function of MocuFH1, gastrulation movements were inhibited (Olsen and Jeffery, 1997). Shimauchi et al. (2001a) suggested a role of FoxA5 also in notochord formation.
Endoderm precursors are formed from vegetal pole blastomeres (Fig. 1). At the 32-cell stage, expression of a LIM-class homeobox gene, Lhx3, starts (Wada et al., 1995). Expression of the gene is not strictly restricted to endoderm blastomeres at first, but then becomes restricted to endoderm blastomeres by the 110-cell stage (Fig. 4). Satou et al. (2001a) showed that Lhx3 is required and sufficient for expression of late marker genes of endoderm differentiation. Overexpression of Lhx3 promotes ectopic endoderm formation without À-catenin activity. Therefore, Lhx3 would be a key zygotic transcription factor for endoderm formation. At the 64-cell stage, the expression of TTF1 is initiated exclusively in endoderm precursors (Fig. 4). TTF1 is a transcription factor containing an NK-2-like homeodomain. When synthetic mRNA of TTF1 is injected into eggs, ectopic endoderm cells are formed (Ristoratore et al., 1999; Satou et al., 2001a). However, injection of its MO did not affect endoderm differentiation (Satou et al., 2001a), although the efficiency of the MO needs to be confirmed. GATA3/4/5 and Sox17 genes have been reported to play important roles in endoderm formation in various metazoans (Laverriere et al., 1994; Hudson et al., 1997). However, these genes do not seem to be significant in early endoderm specification processes in ascidians so far. Thus, À-catenin nuclear localization promotes expression of some zygotic genes that are involved in gastrulation and specification of endoderm precursors.
In the animal hemisphere, various genes also start zygotic expression during cleavage stages (e.g. Epi-C in Fig. 4; Ishida et al., 1996; Miya and Nishida, 2002). However, much attention has not been paid to the mechanisms of fate specification of epidermis cells. Thus, the relationship of these zygotic genes to À-catenin nuclear localization and their functions in epidermal development have not been analyzed. Key transcription factors in epidermal formation are not yet known.
¢Back to CONTENTSCELL INTERACTIONS AND ZYGOTIC GENE EXPRESSION ALONG THE ANTERO-POSTERIOR AXIS
Induction of Notochord and MesenchymeEndoderm blastomeres are derived from the most vegetal region. Mesoderm and nerve cord precursors surround the central endoderm blastomeres. This marginal area in the vegetal hemisphere shows high complexity and remarkable asymmetry along the A?P axis (Fig. 1). The maternal factors in the PVC play crucial roles in making this region asymmetric. In addition, inductive cell interactions contribute to generating cell diversity in this area. The importance of cell interactions has been revealed by blastomere isolation and recombination, and with inhibitors of cell signaling (reviewed by Nishida, 2002a, b and references therein). Thus, it is now realized that the development of ascidian embryos is not entirely mosaic as has been hitherto thought. Key terms to understand what happens in this area are eFGF signalingf, edirected signaling and asymmetric divisionf and ecell responsivenessf.
FGF signalingIn the 32-cell embryos, central endoderm blastomeres are flanked by a single circle of marginal blastomeres (Fig. 1). The endoderm blastomeres emit an inductive signal towards these marginal cells. FGF (an Fgf9/16/20 gene product) plays a major role as the signaling molecule (Nakatani et al., 1996; Kim et al., 2000; Satou et al., 2002c; Imai et al., 2002a). Treatment of isolated blastomeres with recombinant FGF protein efficiently mimics the normal cell responses. Fgf9/16/20 MO suppresses the inductions. Expression of the Fgf9/16/20 gene starts from the 16-cell stage (Fig. 4) and occurs downstream of À-catenin (Imai et al., 2002a). Thus, acquisition of the inducing ability depends on the position of blastomeres along the A?V axis. The inductive interactions take place at the 32-cell stage. Results of experiments involving recombination of isolated blastomeres at various stages and of experiments determining the periods of sensitivity to FGF treatment and to signaling inhibitors all support the idea that major processes of induction take place at the 32-cell stage (Nakatani et al., 1996; Kim and Nishida, 2001). The expression of Fgf9/16/20 occurs at the right time and in the right place. The signal-receiving blastomeres quickly lose their competence at the next cleavage (Nakatani and Nishida, 1999).
Directed Signaling and Asymmetric DivisionThen asymmetric cell divisions occur in the marginal blastomeres. These blastomere divide radially so that two lines of blastomeres encircle the endoderm blastomeres at the 64-cell stage (Fig. 1). Four anterior blastomeres of the 32-cell embryo divide into four outer nerve cord precursors (colored pale blue in Fig. 1) and four inner notochord precursors (pink) of the 64-cell embryo. (The central nervous system of ascidian tadpole larvae consists of an anterior brain in the cranial region and a posterior nerve cord in the trunk and tail region [Fig. 1]. Posterior-nerve cord precursors are blastomeres in the vegetal hemisphere and give rise to the posterior neural tube in the trunk and tail region.) Similarly in the posterior region, four blastomeres of the 32-cell embryo divide into four outer muscle precursors (red) and four inner mesenchyme precursors (dark green). In these processes, outer nerve cord and muscle fates represent the default state, and inner notochord and mesenchyme fates represent the induced fates. When the relevant blastomeres are isolated before induction at the 32-cell stage or treated with signaling inhibitors, both daughters of them assume the default fate. In contrast, when isolated blastomeres are treated with FGF dissolved in seawater, both daughters take the induced fate (Kim et al., 2000; Minokawa et al., 2001).
A conspicuous feature of these inductions is the asymmetric cell divisions of the cells that received the inductive signal. On the basis of the results described above, it is possible to imagine the following scenario. In normal embryos, the precursor cells of the 32-cell embryo receive an endoderm signal from the vegetal pole, and only one of the daughter cells facing the endoderm assumes an induced cell fate in the 64-cell embryo. Directed signals that emanate from endoderm blastomeres may polarize the responding blastomeres at the 32-cell stage and promote asymmetric divisions that operate in both the anterior and posterior regions. This directed signaling and asymmetric division model is supported by the fact that treatment of isolated blastomeres with FGF in seawater causes both daughters to assume induced fates, because isolated mother blastomeres receive the signal over the entire cell surface (Kim et al., 2000; Minokawa et al., 2001). The results of blastomere transplantation also support the model (Kim G. J. and HN, unpublished). Presumably, FGF signaling causes localized changes in the mother cell. The extracellular FGF signal is transduced to the nuclei by conserved molecules in ascidians: FGF receptor, ras, MEK, and MAPK (Nakatani and Nishida, 1997; Kim and Nishida, 2001; Shimauchi et al., 2001b). The mRNAs of these genes are supplied maternally. The simplest model is that MAPK is activated in one side of the signal-receiving cell that faces the inducer cell, and then cleavage starts before the activation propagates into the entire cytoplasmic region of the cell. At least, activated and phosphorylated MAPK is observed only in the nucleus of one daughter cell that faces the endoderm (Nishida, 2003), although a high background of immunostaining unfortunately prevented detection of asymmetric activation of MAPK in the cytoplasm of the mother cells before the asymmetric cell division.
Responsiveness to inductive signalEts family transcription factors is known as one of the targets of the Ras?MAPK signaling pathway (reviewed by Wasylyk et al., 1998; Yordy and Muise-Helmericks, 2000). It has been shown that Ets is also required for notochord and mesenchyme induction in ascidian (Miya and Nishida, 2003). Ets mRNA is maternally present and ubiquitous. The same FGF signal and the same intracellular signal transduction cascade down to the Ets transcription factor induce different responses in the anterior and posterior marginal blastomeres. What causes this difference in responsiveness? The difference must preexist within the responding cells. Results of removal and transfer of egg cytoplasm indicate that the PVC is involved. Indeed, macho-1 causes the difference between anterior and posterior blastomeres (Kobayashi et al., 2003). macho-1-deficient embryos form notochord in the posterior region in place of mesenchyme. macho-1-overexpressed embryos form mesenchyme in place of notochord. Most intriguing result is that when macho-1 is overexpressed in entire embryos and blastomeres of the animal hemisphere are isolated and treated with FGF, those cells develop into mesenchyme. macho-1 is sufficient to confer competence for induction by FGF to form mesenchyme even in animal blastomeres. Therefore, without macho-1, the anterior marginal blastomeres divide asymmetrically into notochord and nerve cord precursors. The posterior marginal blastomeres containing macho-1 divide into mesenchyme and muscle. In inductive interactions, extracellular signaling molecule and intrinsic factors are combined in signal-receiving cells, conferring particular cell fates. Intrinsic factors of responding cells play roles in defining the response. Thus, in the case of these ascidian embryonic inductions, the extracellular signal is transduced into the Ets transcription factor, while the intrinsic competence factor is macho-1 transcription factor.
Zygotic Expression of Key Transcription Factors ? Muscle and Notochordmacho-1 is a transcription activator and binds to specific DNA sequences. Yagi et al. (2004) and Sawada et al. (2005) have reported genes downstream of macho-1. Various genes for transcription factors and muscle structural protein have been found. Some of these genes have a macho-binding consensus sequence in their 5? upstream regions (Yagi et al., 2004). Among these genes, Tbx6 is a candidate of a key transcription factor for muscle development, because misexpression of Tbx6 causes ectopic expression of muscle structural genes (Mitani et al., 1999). Tbx6 is expressed in muscle precursor blastomeres at cleavage stages (Fig. 4). The expression of a myogenic factor (AMD1 in Halocynthia, Ci-MDF in Ciona; Satoh et al., 1996; Meedel et al., 1997) is also muscle-specific at the cleavage stages, although the function of it is not fully analyzed yet. On the other hand, twist-like1 is a key transcription factor for mesenchyme differentiation; it is specifically expressed in mesenchyme precursors. The expression depends on macho-1 and FGF, and twist-like1 is essential for the expression of late mesenchyme marker genes (Fig. 4, Imai et al., 2003; Tokuoka et al., 2004). snail also lies downstream of macho-1 (Kobayashi et al., 2003), and is involved in the control of responsiveness. snail encodes a Zn-finger transcription repressor and is expressed in muscle and mesenchyme precursors at the 32-cell stage (Fig. 4, Erives et al., 1998; Wada and Saiga, 1999). As discussed two paragraphs below, brachyury is a key transcription factor for notochord differentiation in ascidians. Analysis of the cis-regulatory region of the brachyury gene and overexpression of snail mRNA indicate that snail represses the activation of brachyury in the posterior blastomeres (Fujiwara et al., 1998; Kobayashi et al., 2003). This partly explains how the macho-1 transcription activator can suppress notochord fate in the posterior region.
Antibodies against macho-1 protein are not available yet, but evidence is accumulating that macho-1 translation is initiated at the 8-cell stage, and the protein diffuses into the entire cytoplasm of the posterior-vegetal (B4.1) blastomeres of the 8-cell embryo. The B4.1 blastomere gives rise mainly to muscle, mesenchyme, and endoderm. However, every descendant of the B4.1 blastomere, even endoderm-lineage cells, assumes muscle fate without cell interaction (Kondoh et al., 2003). FGF and BMP are involved in formation of endoderm cells from the B4.1 blastomeres. By contrast, endoderm formation from the A4.1 (anterior-vegetal) blastomeres, which do not have macho-1 products, is a cell-autonomous process and does not require cell interaction. The cell interactions in the B4.1 descendants are necessary for the suppression of macho-1 function in B-line endoderm precursors, because injection of macho-1 MO makes cell interaction unnecessary (Kondoh et al., 2003). Therefore, macho-1 directs muscle fate in posterior muscle cells; its function is likely modified by FGF signaling to promote mesenchyme fate in intermediate mesenchyme cells; and in central B-line endoderm cells its function is totally suppressed by FGF and BMP. Probably there are intrinsic differences in responsiveness to FGF between mesenchyme and endoderm blastomeres, and the localization of endoderm determinants could account for the differences. The cell interactions would restrict the regions where maternal determinants work, and play a role in marking a precise boundary between precursor cells of different tissue types. It would be hard for embryos to precisely partition muscle determinants exclusively into multiple muscle precursor blastomeres through a complicated cleavage history. These findings suggest that the cell interactions are also utilized to suppress functions of inappropriately distributed maternal determinants after embryogenesis starts.
FGF treatment can induce notochord fate only in presumptive notochord/nerve cord-lineage cells. Cells in the animal hemisphere never respond to FGF by assuming notochord fate. This indicates that responsiveness to induction to form notochord is restricted to marginal notochord/nerve cord-lineage cells. À-catenin-deficient embryos fail to develop notochord, as the downstream FGF9/16/20 is not expressed. However, even if FGF9/16/20 is overexpressed in À-catenin-deficient embryos, notochord is not formed (Imai et al., 2000; 2002a). This indicates that competence to be induced to form notochord is also lost. It has been suggested that À-catenin, FoxD, and Zic are involved in the acquisition of competence (Fig. 4). MOs of these genes abolish notochord. FoxD gene is a direct target of À-catenin, as its expression depends on À-catenin and on a Tcf-binding site in its 5? upstream cis-element (Imai et al., 2002b). FoxD expression is independent of FGF9/16/20 and vice versa. Expression of FoxD is transient at the 16-and 32-cells stages in the vegetal hemisphere. FoxD is expressed in notochord/nerve cord/endoderm-lineage blastomeres at the 16-cell stage. Then Zic expression starts in notochord/nerve cord-lineage cells at the 32-cell stage (Wada and Saiga, 2002; Imai et al., 2002c). The expression depends on FoxD and persists in both notochord and nerve cord precursors at the 64-cell stage. Finally, expression of brachyury, a key transcription factor in notochord formation, specifically starts in notochord precursors of 64-cell embryos (Yasuo and Satoh, 1998). Injection of Zic MO eliminates brachyury expression and notochord formation (Wada and Saiga, 2002; Imai et al., 2002c), and Zic overexpression ectopically activates the brachyury promoter through the Zic-binding site (Yagi et al., 2003). Zic appears to be a competence factor in notochord induction, although this needs further investigation. Zic contains a Zn-finger domain that is closely related to that of macho-1. Therefore, it is interesting that similar Zn-finger proteins with the same origin are utilized in anterior (Zic) and posterior (macho-1) regions. Another interesting symmetry is seen in the key transcription factors. Both brachyury and Tbx6 belong to the T-box family, and they participate in notochord (anterior) and muscle (posterior) formation, respectively.
Zygotic Expression of Key Transcription Factors ? Mesenchyme, TVC, and TLCAmong mesodermal tissues, muscle and notochord function at the swimming tadpole stage. In addition, three cell types in the larvae?mesenchyme, trunk lateral cells (TLCs), and trunk ventral cells (TVCs)?are preserved in the larvae and function after metamorphosis. Mesenchyme gives rise to tunic cells in the juvenile. TLCs are precursors of blood cells and body wall muscle. TVCs develop into heart and body wall muscle (Hirano and Nishida, 1997). As mentioned above, mesenchyme specification requires macho-1 and FGF. Twist-like1 is a key transcription factor for mesenchyme. Recent studies have shown that TLCs and TVCs are specified during cleavage stages. TLC precursor (A7.6) blastomeres of the 64-cell embryo form in the lateral region of the vegetal hemisphere (sky blue in Fig. 1). Hand-like/NoTrlc is a key transcription factor for TLC formation (Imai et al., 2003). It has a bHLH domain and is specifically expressed in TLC precursors from the 64-cell stage (Fig. 4). FoxD activity is required for the expression of the Hand-like gene. Blastomere isolation and recombination experiments showed that inductive interaction from the animal blastomere at the 16-cell stage is important, and that only TLC-lineage blastomeres have competence (Kawaminami and Nishida, 1997).
TVC precursor blastomeres (B7.5) and putative germline precursor blasmtomeres (B7.6) are present in the posterior-most region (purple and brown in Fig. 1, respectively). B7.5 and B7.6 are sister cells that are generated by the final unequal cleavage to the 64-cell stage. B7.6 is the smaller blastomere; it is present at the posterior pole and inherits the CAB and postplasmic/PEM RNAs as mentioned before. On the other hand, B7.5 is precursor of TVCs of the larvae, and adult heart. Mesp, which has a bHLH domain, is a key transcription factor for formation of TVCs and adult heart. It is specifically expressed in TVC precursors from the 110-cell stage (Fig. 4). macho-1 is required for Mesp expression (Satou et al., 2004). In accordance, every descendant of B7.5 assumes larval muscle fate without cell interaction (Nishida, 1992; Kondoh et al., 2003). In more detail, the B7.5 blastomere divides into a larval muscle precursor and a TVC precursor at the next division. Therefore, directed signaling and asymmetric division might also operate in this cell division (fig. 1). Various cell interactions are speculated to be involved in TVC specification (Davidson and Levine, 2003). The competence factor for TVC induction may be one of the postplasmic/PEM RNAs.
The marginal zone of the vegetal hemisphere is patterned along the A?P axis, as discussed above. Localized maternal factors are involved in the A?P polarization. Then cellular interactions play important roles in this patterning when embryos become multicellular. Zygotic gene expression is involved in production of both of inductive signals and competence factors, and finally expression of cell-type specific key transcription factors is bought about.
Animal HemisphereWhat happens in the animal hemisphere is much simpler. Most regions of the animal hemisphere are occupied by epidermis blastomeres. Only on the anterior edge are brain precursors formed (Fig. 1). The formation of brain precursors requires induction from the vegetal blastomeres (reviewed by Okamura et al., 1993; Lemaire et al., 2002; Meinertzhagen et al., 2004). Otx, a putative key transcription factor for brain development, is expressed in these blastomeres (Fig. 4). Otx is a homeobox transcription factor that plays important roles in brain development in Drosophila and in vertebrates (Simeone, 1998). The functions of otx have been investigated with MO (Wada et al., 2004). In the absence of Otx function, brain development was abrogated, but expression of some brain-specific genes persisted. Therefore, the idea that Otx is a key transcription factor is still controversial. FGF/Ets and GATA are crucial for the expression in brain precursors (Inazawa et al., 1998; Miya and Nishida, 2003; Bertrand et al., 2003). In accordance, Ets- and GATA-binding cis-elements are crucial for the expression of the Otx?reporter construct. Thus, the FGF signal from the vegetal hemisphere induces brain precursors through activation of Ets. GATA gene expression is maternal and ubiquitous, but it is translated only in animal blastomeres at the late 32-cell stage, when induction takes place. These results suggest that GATA is the competence factor that restricts brain induction to the animal hemisphere (Bertrand et al., 2003). It is still not clear how the brain is induced only at the anterior edge, although blastomere recombination experiments and treatment with inducing substance demonstrated that the anterior and posterior blastomeres have different efficiencies of brain induction (Okado and Takahashi, 1993; Hadson and Lemaire, 2001).
¢Back to CONTENTSSUMMARY AND FUTURE DIRECTIONS
Mosaic Development and Cell InteractionsAs can be seen in Figs. 1, 3, and 4, fate specification and gene expression have been analyzed blastomere by blastomere at the single-cell level in the ascidian embryo. This combined with the stereotypic cleavage pattern makes studies of ascidian embryogenesis easy to analyze and understand. Ascidian embryogenesis has been hitherto regarded as a typical example of mosaic development, but recent analyses revealed that cell interactions play equally important roles. In retrospect, there are two main reasons for ascidian embryos to be regarded as a mosaic. When blastomeres are isolated at an early cleavage stage, for example at the 8-cell stage, cell interactions take place within the partial embryos derived from the isolated blastomeres. Therefore, the blastomeres can develop various tissues as expected from the fate map, and spuriously behave in an autonomous way. In other words, the blastomeres can develop autonomously in isolation, however, tissue formation is not cell-autonomous process in these cases.
The second reason is that ascidian embryos do not compensate for lost parts if blastomeres are ablated. This is because competence or responsiveness to be induced to certain tissues is indeed restricted to the tissue precursor blastomeres, as discussed in this article. Generally in animal development, various organisms utilize a limited repertoire of signaling molecules for intercellular communication. The same signals are used over and over again in different cells at different stages, with different biological outcomes depending on the spatial and temporal context. To understand animal development, it is important to clarify how embryonic cells can respond differently to the same signal, and to understand how competence is restricted to specific cells. Ascidian embryo provides a good model for elucidating this issue. Cellular simplicity and transparence of ascidian embryos have opened the way to the use of sophisticated labeling and imaging techniques, which allow for 3 D and 4 D reconstruction of virtual embryos. Hence, the imaging tools may help access to cell contact surface or cell division features of neighboring blastomeres. Ascidian embryo is indeed a perfect system for elucidating cellular communication at early developmental stages because of its limited cell number and invariant mode of development.
Maternal FactorsAnalysis of maternal processes is also underway and the most pressing questions are: How is the A?V polarity established during oogenesis? Is Dishevelled a vegetal determinant in ascidians? Is there a gradient of À-catenin stabilization along the A?V axis, and how is the intermediate marginal zone specified? What are the roles of unanalyzed postplasmic/PEM RNAs in establishment of the A?P axis? Cellular mechanisms of unequal cleavage in relation to the CAB and anchoring of postplasmic RNA to cER will be important issues to pursue. In addition, spatio-temporal analysis of FGF signal transduction processes will be required to fully understand the mechanisms of asymmetric divisions after cells receive directed signals.
Genome and ESTsAn evolutionary point of view is also interesting because of the phylogenic position of ascidians (Di Gregorio and Levine, 2001; Corbo et al., 2001; Satoh, 2003a). So far, most research has been concentrated on embryogenesis. However, analysis of mechanisms of metamorphosis is also worth analysis (Hirano and Nishida, 1997, 2000; Eri et al., 1999; Davidson and Swalla, 2002).
Recently, infrastructure for ascidian research has been expeditiously set up. A cDNA project involving large-scale ESTs and in situ hybridization of maternal mRNA in Halocynthia roretzi (http://www.genome.jp/magest/; Makabe et al., 2001) and a cDNA project on maternal and zygotic transcripts in Ciona intestinalis (http://ghost.zool.kyoto-u.ac.jp/indexr1.html; Satou et al., 2001b; Satou et al., 2002b) will help future studies. The genome sequence of C. intestinalis is completed (http://genome.jgi-psf.org/ciona4/ciona4.home.html; Dehal et al., 2002; Satoh, 2003b) and that of C. savignyi is nearly completed (http://www.broad.mit.edu/annotation/ciona/; http://www2.bioinformatics.tll.org.sg:8082/Ciona_savignyi/). Comparison of promoter sequences in these closely related species are useful for detecting conserved regulatory cis-elements for gene expression (Johnson et al., 2004).
Developmentally important genes are annotated in a special ascidian issue of Development, Genes and Evolution (Vol. 213, No. 5?6; 2003). Recently, Imai et al. (2004) listed every gene for transcription factors and signaling molecules in the C. intestinalis genome, and the expression pattern during embryogenesis was comprehensively reported (http://ghost.zool.kyoto-u.ac.jp/tfst.html). The minimal gene redundancy in the ascidian genome will simplify our understanding of the expression patterns and the results of gene knockdown experiments. The wealth of genetic information on ascidians will promote studies to envisage the molecular network during ascidian embryogenesis. Genetic approaches are now feasible in Ciona and have begun bearing fruit (e.g. Nakatani et al., 1999; Awazu et al., 2004). Another possible genetic approach will be to utilize appendicularians (Urochordata, a larvecian, Oikopleura), which have a simple chordate body plan (Fenaux, 1998; Nishino and Satoh, 2001), and their life cycle is only four to seven days.
¢Back to CONTENTSREFERENCES
Arendt D, Nubler-Jung K. 1997. Dorsal or ventral: similarities in fate maps and gastrulation patterns in annelids, arthropods and chordates. Mech Dev 61:7-21.Awazu S, Sasaki A, Matsuoka T, Satoh N, Sasakura Y. 2004. An enhancer trap in the ascidian Ciona intestinalis identifies enhancer of its Musashi orthologous gene. Dev Biol 275:459-472.
Bates WR, Jeffery WR. 1987. Alkaline phosphatase expression in ascidian egg fragments and andromerogons. Dev Biol 119:382-389.
Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. 2003. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell 115:615-627.
Cadigan KM, Nusse R. 1997. Wnt signaling: a common theme in animal development. Genes Dev 11:3286-3305.
Chabry L. 1887. Contribution l'embryologie normale et teratologique des Ascidies simples. J Anat Physiol (Paris) 23:167-319.
Conklin EG. 1905. The organization and cell lineage of the ascidian egg. J Acad Nat Sci (Philadelphia) 13:1-119.
Corbo J, Di Gregorio A, Levine M. 2001. The ascidian as a model organism in developmental and evolutionary biology. Cell 106:535-538.
Davidson B, Levine M. 2003. Evolutionary origins of the vertebrate heart: Specification of the cardiac lineage in Ciona intestinalis. Proc Natl Acad Sci 100:11469-11473.
Davidson B, Swalla BJ. 2002. A molecular analysis of ascidian metamorphosis reveals activation of an innate immune response. Development 129:4739-4751.
Dehal P, Satou Y, Campbell RK. et al. 2002. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 298:2157-2167.
Di Gregorio A, Corbo JC, Levine M. 2001. The regulation of forkhead/HNF-3? expression in the Ciona embryo. Dev Biol 229:31-43.
Di Gregorio A, Levine M. 2001. Ascidian embryogenesis and the origins of the chordate body plan. Curr. Opin. Genet Dev 8:457-463.
Emily-Fenouil F, Ghiglione C, Lhomond G, Lepage T, Gache C. 1998. GSK3?/shaggy mediates patterning along the animal-vegetal axis of the sea urchin embryo. Development 125:2489-2498.
Eri R, Arnold JM, Hinman VF, Green KM, Jones MK, Degnan BM, Lavin MF. 1999. Hemps, a novel EGF-like protein, plays a central role in ascidian metamorphosis. Development 126:5809-5818.
Erives A, Corbo JC, Levine M. 1998. Lineage-specific regulation of the Ciona snail gene in the embryonic mesoderm and neuroectoderm. Dev Biol 194:213-225.
Fenaux R. 1998. Life history of the appendicularia. In gThe Biology of Pelagic Tunictesh ed. Q. Bone, Oxford University Press, New York.
Fujimura M, Takamura K. 2000. Characterization of an ascidian DEAD-box gene, CiDEAD1: specific expression in the germ cells and its mRNA localization in the posterior-most blastomeres in early embryos. Dev Genes Evol 210:64-72.
Fujiwara S, Corbo JC, Levine M. 1998. The snail repressor establishes a muscle/notochord boundary in the Ciona embryo. Development 125:2511-2520.
Gilbert SF. 2003. "Developmental Biology." 7th edition. Sinauer Associates, Sunderland. Heasman J. 1997. Patterning Xenopus blastula. Development 124:1553-1560.
Hibino T, Nishikata T, Nishida H. 1998. Centrosome-attracting body: A novel structure closely related to unequal cleavages in the ascidian embryo. Dev Growth Differ 40:85-95.
Hirano T, Nishida H. 1997. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. I. Origin of mesodermal tissues of the juvenile. Dev Biol 192:199-210.
Hirano T, Nishida H. 2000. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. II. Origin of endodermal tissues of the juvenile. Dev Gene Evol 210:55-63.
Hudson C, Lemaire P. 2001. Induction of anterior neural fates in the ascidian Ciona intestinalis. Mech Dev 100:189-203.
Hudson C, Clements D, Friday RV, Stott D, Woodland HR. 1997. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell 91:397-405.
Ikenishi K. 1998. Germ plasm in Caenorhabditis elegans, Drosophila and Xenopus. Dev Growth Differ 40:1-10.
Imai KS. 2003. Isolation and characterization of ?-catenin downstream genes in early embryos of the ascidian Ciona savignyi. Differentiation 71:346-360.
Imai K, Takada N, Satoh N, Satou Y. 2000. ?-catenin mediates the specification of endoderm cells in ascidian embryos. Development 127:3009-3020.
Imai KS, Satoh N, Satou Y. 2002a. Early embryonic expression of FGF4/6/9 gene and its role in the induction of mesenchyme and notochord in Ciona savignyi embryos. Development 129:1729-1738.
Imai KS, Satoh N, Satou Y. 2002b. An essential role of a FoxD gene in notochord induction in Ciona intestinalis. Development 129:3441-3453.
Imai KS, Satou Y, Satoh N. 2002c. Multiple functions of a Zic-like gene in the differentiation of notochord, central nervous system and muscle in Ciona savignyi embryos. Development 129:2723-2732
Imai KS, Satoh N, Satou Y. 2003. A twist-like bHLH gene is a downstream factor of an endogenous FGF and determines mesenchymal fate in the ascidian embryos. Development 130:4461-4472
Imai KS, Hino K, Yagi K, Satoh N, Satou Y. 2004. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development 131:4047-4058.
Inazawa T, Okamura Y, and Takahashi K. 1998. Basic fibroblast growth factor induction of neural ion channel expression in ascidian ectodermal blastomeres. J Physiol 511:347-359.
Iseto T, Nishida H. 1999. Ultrastructual studies on the centrosome-attracting body: Electron-dense matrix and its role in unequal cleavage in ascidian embryos. Dev Growth Differ 41:601-609.
Ishida K, Ueki T, Satoh N. 1996. Spatio-temporal expression patterns of eight epidermis-specific genes in the ascidian embryo. Zool Sci 13:699-709.
Jeffery WR. 2001. Determinants of cell and positional fate in ascidian embryos. Int Rev Cytol 203:3-62.
Johnson D, Davidson B, Brown CD, Smith WC, Sidow A. 2004. Noncoding regulatory sequences of Ciona exhibit strong correspondence between evolutionary constraint and functional importance. Genome Res in press.
Kawaminami T, Nishida H. 1997. Induction of trunk lateral cells, the blood cell precursors, during ascidian embryogenesis. Dev Biol 181:14-20.
Kim GJ, Yamada A, Nishida H. 2000. An FGF signal from endoderm and localized factors in the posterior-vegetal egg cytoplasm pattern the mesodermal tissues in the ascidian. Development 127:2853-2862.
Kim GJ, Nishida H. 2001. Role of FGF and MEK signaling pathway in the ascidian embryo. Dev Growth Differ 43:521-533.
Kobayashi K, Sawada K, Yamamoto H, Wada S, Saiga H, Nishida H. 2003. Maternal macho-1 is an intrinsic factor that makes cell response to the same FGF signal differ between mesenchyme and notochord induction in ascidian embryos. Development 130:5179-5190.
Kondoh K, Kobayashi K, Nishida H. 2003. Suppression of macho-1-directed muscle fate by FGF and BMP is required for formation of posterior endoderm in ascidian embryos. Development 130:3205-3216.
Laverriere AC, MacNiell C, Mueller C, Poelmann RE, Burch JBE, Evance T. 1994. GATA-4/5/6: a subfamily of three transcription factors transcribed in the developing heart and gut. J Biol Chem 269:23177-23184.
Lemaire P, Bertrand V, Hudson C. 2002. Early steps in the formation of neiral tissue in ascidian embryos. Dev Biol 252:151-169.
Logan CY, Miller JR, Ferkowicz MJ, MaClay DR. 1999. Nuclear ?-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126:345-357.
Makabe KW, Kawashima T, Kawashima S, et al. 2001. Large-scale cDNA analysis of the maternal genetic information in the egg of the ascidian, Halocynthia roretzi, for the gene expression catalog during development. Development 128:2555-2567.
Meedel TH, Farmer SC, Lee JJ. 1997. The single MyoD family genes of Ciona intestinalis encodes two differentially expressed proteins: implications for the evolution of chordate muscle gene regulation. Development 124:1711-1721.
Meinertzhagen IA, Lemaire P, Okamura Y. 2004. The neurobiology of the ascidian tadpole larva: Recent developments in an ancient chordates. Annu. Rev Neurosci 27:453-485.
Miller JR, Moon RT. 1996. Signal transduction through ?-catenin and specification of cell fates during embryogenesis. Genes Dev 10:2527-2539.
Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. 1999. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol 146:427-437.
Minokawa T, Yagi K, Makabe KW, Nishida H. 2001. Binary specification of nerve cord and notochord cell fates in ascidian embryos. Development 128:2007-2017.
Mitani Y, Takahashi H, Satoh N. 1999. An ascidian T-box gene As-T2 is related to the Tbx6 subfamily and is associated with embryonic muscle cell differentiation. Dev Dyn 215:62-68. Miya T, Nishida H. 2002. Isolation of cDNA clones for mRNAs transcribed zygotically during cleavage in the ascidian, Halocynthia roretzi. Dev Genes Evol 212:31-37.
Miya T, Nishida H. 2003. An Ets transcription factor, HrEts, is target of FGF signaling and involved in induction of notochord, mesenchyme, and brain in ascidian embryos. Dev Biol 261:25-38.
Nakamura Y, Makabe KW, Nishida H. 2003. Localization and expression pattern of type I postplasmic mRNAs in embryos of the ascidian Halocynthia roretzi. Gene Express Pattern 3:71-75.
Nakatani Y, Yasuo H, Satoh N, Nishida H. 1996. Basic fibroblast growth factor induces notochord formation and the expression of As-T, a Brachyury homolog, during ascidian embryogenesis. Development 122:2023-2031.
Nakatani Y, Nishida H. 1997. Ras is an essential component for notochord formation during ascidian embryogenesis. Mech Dev 68:81-89.
Nakatani Y, Nishida H. 1999. Duration of competence and inducing capacity of blastomeres in notochord induction during ascidian embryogenesis. Dev Growth Differ 41:449-453.
Nakatani Y, Moody R, Smith WC. 1999. Mutations affecting tail and notochord development in the ascidian Ciona savignyi. Development 126:3293-3301.
Nieuwkoop PD. 1977. Origin and establishment of embryonic polar axes in amphibian development. Curr Top Dev Biol 11:115-132.
Nishida H. 1987. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue-restricted stage. Dev Biol 121:526-541.
Nishida H. 1992a. Developmental potential for tissue differentiation of fully dissociated cells of the ascidian embryo. Roux's Arch Dev Biol 201:81-87.
Nishida H. 1992b. Regionality of egg cytoplasm that promotes muscle differentiation in embryo of the ascidian, Halocynthia roretzi. Development 116:521-529.
Nishida H. 1994. Localization of determinants for formation of the anterior-posterior axis in eggs of the ascidian Halocynthia roretzi. Development 120:3093-3104.
Nishida H. 1996. Vegetal egg cytoplasm promotes gastrulation and is responsible for specification of vegetal blastomeres in embryos of the ascidian Halocynthia roretzi. Development 122:1271-1279.
Nishida H. 1997. Cell-fate specification by localized cytoplasmic determinants and cell interaction in ascidian embryos. Int Rev Cytol 176:245-306.
Nishida H. 2002a. Specification of developmental fates in ascidian embryos: Molecular approach to maternal determinants and signaling molecules. Int Rev Cytol 217:227-276.
Nishida H. 2002b. Patterning the marginal zone of early ascidian embryos: Localized maternal mRNA and inductive interactions. BioEssays 24:87-92.
Nishida H. 2003. Spatio-temporal pattern of the activation of MAP kinase in embryos of the ascidian, Halocynthia roretzi. Dev Growth Differ 45:27-37.
Nishida H, Morokuma J, Nishikata T. 1999. Maternal cytoplasmic factors for generation of unique cleavage pattern in animal embryos. Cur Top Dev Bio 46:1-37.
Nishida H, Sawada K. 2001. macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature 409:724-729.
Nishikata T, Hibino T, Nishida H. 1999. The centrosome-attracting body, microtubule system, and posterior egg cytoplasm are involved in positioning of cleavage planes in the ascidian embryo. Dev Biol 209:72-85.
Nishino A, Satoh N. 2001. The simple tail of chordates: Phylogenic significance of appendicularians. Genesis 29:36-45.
Numakunai T. 2001. Oocyte maturation and self-sterility by treatment with ovary extracts of the ascidian, Halocynthia roretzi. In gThe Biology of Ascidiansh Sawada H, Yokosawa H, Lambert CC. eds. Springer-Verlag, Tokyo.
Okado H, Takahashi K. 1993. Neural differentiation in cleavage-arrested ascidian blastomeres induced by a proteolytic enzyme. J Physiol 463:269-290.
Okamura Y, Okado H, Takahashi K. 1993. The ascidian embryo as a prototype of vertebrate neurogenesis. BioEssays 15:723-729.
Olsen CL, Jeffery WR. 1997. A forkhead gene related to HNF-3b is required for gastrulation and axis formation in the ascidian embryo. Development 124:3609-3619.
Reverberi G, Minganti A. 1946. Fenomeni di evocatione nelle sviluppo dell'uovo di ascidie. Pubbl Stn Zool Napoli 20:199-252.
Ristoratore F, Spagnuolo A, Aniello F, Branno M, Fabbrini F, Di Lauro R. 1999. Expression and functional analysis of Cititf1, an ascidian NK-2 class gene, suggest its role in endoderm development. Development 126:5149-5159.
Roegiers F, McDougall A, Sardet C. 1995. The sperm entry point defines the orientation of the calcium-induced contraction wave that directs the first phase of cytoplasmic reorganization in the ascidian egg. Development 121:3457-3466.
Roegiers F, Djediat C, Dumollard R, Rouviere C, Sardet C. 1999. Phases of cytoplasmic and cortical reorganizations of the ascidian zygote between fertilization and first division. Development 126:3101-3117.
Sardet C, Speksnijder J, Inoue S, Jaffe L. 1989. Fertilization and ooplasmic movements in the ascidian egg. Development 105:237-249.
Sardet C, Speksnijder J, Terasaki M, Chang P. 1992. Polarity of the ascidian egg cortex before fertilization. Development 115:221-237.
Sardet C, McDougall, Houliston E. 1994. Cytoplasmic domains in eggs. Trends Cell Biol 4:166-172.
Sardet C, Prodon F, Dumollard R, Chang P, Chenevert J. 2002. Structure and function of the egg cortex from oogenenesis through fertilization. Dev Biol 241:1-23.
Sardet C, Nishida H, Prodon F, Sawada K. 2003. Maternal mRNAs of PEM and macho-1, the ascidian muscle determinant, associate and move with a rough endoplasmic reticulum network in the egg cortex. Development 130:5839-5849.
Sardet C, Dru P, Prodon F. 2005. Maternal determinants and mRNA in the cortex of ascidian oocytes, zygotes and embryos. Biol Cell 97:1-15.
Sasakura T, Makabe KW. 2002. Identification of cis elements which direct the localization of maternal mRNAs to the posterior pole of ascidian embryos. Dev Biol 250:128-144.
Sasakura Y, Ogasawara M, Makabe KW. 1998a. HrWnt-5: a maternally expressed ascidian Wnt gene with posterior localization in early embryos. Int J Dev Biol 42:573-579.
Sasakura Y, Ogasawara M, Makabe KW. 1998b. Maternally localized RNA encoding a serine/threonine protein kinase in the ascidian, Halocynthia roretzi. Mech Dev 76:161-163.
Sasakura Y, Ogasawara M, Makabe KW. 2000. Two pathways of maternal RNA localization at the posterior-vegetal cytoplasm in early ascidian embryos. Dev Biol 220:365-378.
Satoh N. 1994. "Developmental Biology of Ascidians." Cambridge University Press, Cambridge.
Satoh N. 2003a. The ascidian tadpole larva: comparative molecular development and genomics. Nature Rev Genet 4:285-295.
Satoh N. 2003b. Ciona intestinalis: an emerging model for whole-genome analysis. Trends Genet 19:361-381.
Satoh N, Araki I, Satou Y. 1996. An intrinsic genetic program for autonomous differentiation of muscle cells in the ascidian embryo. Proc Natl Acad Sci USA 93:9315-9321.
Satou Y, Imai KS, Satoh N. 2001a. Early embryonic expression of a LIM-homeobox gene Cs-lhx3 is downstream of ?-catenin and responsible for the emdoderm differentiation in Ciona savignyi embryos. Development 128:3559-3570.
Satou Y, Takatori N, Yamada L. et al. 2001b. Gene expression profiles in Ciona intestinalis tailbud embryos.
Satou Y, Yagi K, Imai KS, Yamada L, Nishida H, Satoh N. 2002a. macho-1-related genes in Ciona embryos. Dev Genes Evol 212:87-92.
Satou Y, Takatori N, Fujiwara S, Nishikata T, Saiga H, Kusakabe T, Shin-i T, Kohara Y, Satoh N. 2002b. Ciona intestinalis cDNA projects: expressed sequence tag analysis and gene expression profiles during embryogenesis. Gene 287:83-96.
Satou Y, Imai KS, Satoh N. 2002c. Fgf genes in the basal chordate Ciona intestinalis. Dev Genes Evol 212:432-438.
Satou Y, Imai KS, Satoh N. 2004. The ascidian Mesp gene specifies heart precursor cells. Development 131:2533-2541.
Sawada T. 1988. The mechanism of ooplasmic segregation in the ascidian eggs. Zool Sci 5:667-675.
Sawada T, Schatten G. 1988. Microtubules in ascidian eggs during meiosis, fertilization, and mitosis. Cell Motil Cytoskeleton 9:219-230.
Sawada K, Fukushima Y, Nishida H. 2005. Macho-1 functions as transcriptional activator for muscle formation in embryos of the ascidian Halocynthia roretzi. Gene Express Pattern 5:429-437.
Schneider S, Steinbesser H, Warga RM, Hausen P. 1996. ?-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57:191-198.
Seydoux G, Dunn MA. 1997. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA Polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 121:2191-2201.
Shimauchi Y, Yasuo H, Satoh N. 1997. Autonomy of ascidian fork head/HNF-3 gene expression. Mech Dev 69:143-154.
Shimauchi Y, Chiba S, Satoh N. 2001a. Synergistic action of HNF-3 and Brachyury in the notochord differentiation of ascidian embryos. Int J Dev Biol 45:643-652.
Shimauchi Y, Murakami SD, Satoh N. 2001b. FGF signals are involved in the differentiation of notochord cells and mesenchyme cells of the ascidian Halocynthia roretzi. Development 128:2711-2721.
Simeone A. (1998). Otx1 and Otx2 in the development and evolution of the mammalian brain. EMBO J 17:6790-6798.
Speksnijder J, Jaffe L, Sardet C. 1989. Polarity of sperm entry in the ascidian egg. Dev Biol 133:180-184.
Takamura K, Fujimura M, Yamaguchi Y. 2002. Primordial germ cells originate form the endodermal strand cells in the ascidian Ciona intestinalis. Dev Genes Evol 212:11-18.
Takatori N, Hotta K, Mochizuki Y, Satoh G, Mitani Y, Satoh N, Satou Y, Takahashi H. 2004. T-box genes in the ascidian Ciona intestinalis: Characterization of cDNAs and spatial expression. Dev Dyna. 230:743-753.
Tokuoka M, Imai KS, Satou Y, Satoh N. 2004. Three distinct lineages of mesenchymal cells in Ciona intestinalis embryos demonstrated by specific gene expression. Dev Bio 274:211-224.
Tomioka M, Miya T, Nishida H. 2002. Repression of zygotic gene expression in the putative germline cells in ascidian embryos. Zool Sci 19:49-55.
Van Doren M, Williamson AL, Lehmann R. 1998. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol 8:243-246.
Wada S, Saiga, H. 1999. Cloning and embryonic expression of Hrsna, a snail family gene of the ascidian Halocynthia roretzi: Implication in the origin of mechanisms for mesoderm specification and body axis formation in chordates. Dev Growth Differ 41:9-18.
Wada S, Saiga H. 2002. HrZicN, a new Zic family genes of ascidians, plays essential roles in the neural tube and notochord development. Development 129:5597-5608.
Wada S, Katsuyama Y, Yasugi S, Saiga H. 1995. Spatially and temporally regulated expression of the LIM class homeobox gene Hrlim suggests multiple distinct function in development of the ascidian, Halocynthia roretzi. Mech Dev 51:115-126.
Wada S, Sudou N, Saiga H. 2004. Roles of HrOth, the ascidian otx gene, in the differentiation of the brain (sensory vesicle) and anterior trunk epidermis in the larval development of Halocynthia roretzi. Mech Dev 121:463-474.
Wasylyk B, Hagman J, Gutierrez-Hartmann A. 1998. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci 23:213-216.
Weitzel HE, Illies MR, Byrum CA, Xu R, Wikramanayake AH, Ettensohn C. 2003. Differential stability of ?-catenin along the animal-vegetal axis of the sea urchin embryo mediated by disheveled. Development 131:2947-1956.
Wikramanayake AH, Huang L, Klein WH. 1998. ?-catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc Natl Acad Sci USA 95:9343-9348.
Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, Xu R, Martindale MQ. 2003. An ancient role for nuclear ?-catenin in the evolution of axial pol rity and germ layer segregation. Nature 426:446-450.
Yagi K, Satou Y, Satoh N. 2003. A zinc finger transcription factor, ZicL, is a direct activator of Brachyury in the notochord specification of Ciona intestinalis. Development 131:1279-1288.
Yagi K, Satoh N, Satou Y. 2004. Identification of downstream genes of the ascidian muscle determinant gene Ci-macho-1. Dev Biol 274:478-489.
Yasuo H, Satoh N. 1998. Conservation of the developmental role of Brachyury in notochord formation in a urochordate, the ascidian Halocynthia roretzi. Dev Biol 200:158-170.
Yordy JS, Muise-Helmericks RC. 2000. Signal transduction and the Ets family of transcription factors. Oncogene 19:6503-6513.
Yoshida S, Marikawa Y, Satoh N. 1996. posterior end mark, a novel maternal gene encoding a localized factor in the ascidian embryo. Development 122:2005-2012.
Zalokar M, Sardet C. 1984. Tracing of cell lineage in embryonic development of Phallusia Mammillata (Ascidia) by vital staining of mitochondria. Dev Bio 102:195-205.
Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. 1998. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94:515-524.
¢Back to CONTENTS